Page 1 - 3. LESSON NOTES-(CONCEPT OF CATHODE AND ANODE RAYS)
P. 1
SAI International School
Class-IX
Subject- Chemistry
Topic-Structure of atom
Subtopic- Concept of Cathode and anode rays
Concept of Cathode and Anode Rays
Lesson Notes
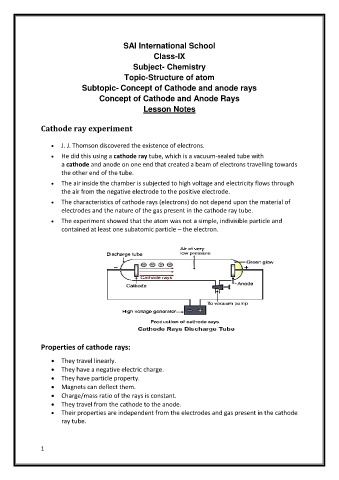
Cathode ray experiment
J. J. Thomson discovered the existence of electrons.
He did this using a cathode ray tube, which is a vacuum-sealed tube with
a cathode and anode on one end that created a beam of electrons travelling towards
the other end of the tube.
The air inside the chamber is subjected to high voltage and electricity flows through
the air from the negative electrode to the positive electrode.
The characteristics of cathode rays (electrons) do not depend upon the material of
electrodes and the nature of the gas present in the cathode ray tube.
The experiment showed that the atom was not a simple, indivisible particle and
contained at least one subatomic particle – the electron.
Properties of cathode rays:
They travel linearly.
They have a negative electric charge.
They have particle property.
Magnets can deflect them.
Charge/mass ratio of the rays is constant.
They travel from the cathode to the anode.
Their properties are independent from the electrodes and gas present in the cathode
ray tube.
1