Page 2 - 3. LESSON NOTES-(MOLE CONCEPT)
P. 2
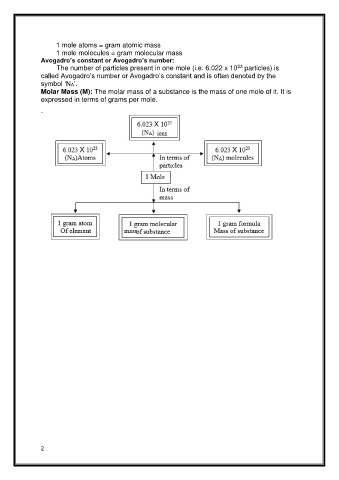
1 mole atoms = gram atomic mass
1 mole molecules = gram molecular mass
Avogadro’s constant or Avogadro’s number:
23
The number of particles present in one mole (i.e. 6.022 x 10 particles) is
called Avogadro’s number or Avogadro’s constant and is often denoted by the
symbol ‘NA’.
Molar Mass (M): The molar mass of a substance is the mass of one mole of it. It is
expressed in terms of grams per mole.
.
2