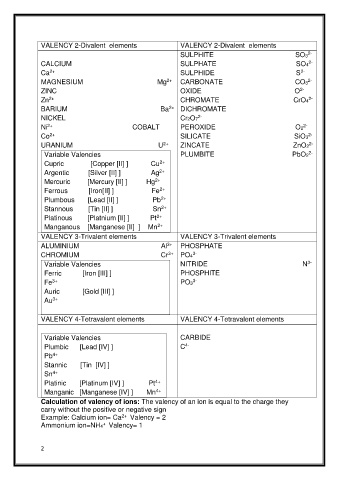
Page 2 - 3. LESSON NOTES-(IONSPOSITIVE&NEGATIVE IONSCALCULATION OF VALENCY OF IONS)
P. 2
VALENCY 2-Divalent elements VALENCY 2-Divalent elements
2-
SULPHITE SO3
2-
CALCIUM SULPHATE SO4
2+
Ca SULPHIDE S
2-
2-
2+
MAGNESIUM Mg CARBONATE CO3
2-
ZINC OXIDE O
2-
2+
Zn CHROMATE CrO4
2+
BARIUM Ba DICHROMATE
2-
NICKEL Cr2O7
IDE
ROX
2+
P
E
Ni COBALT O2
2-
2-
2+
Co SILICATE SiO3
URANIUM U ZINCATE ZnO2
2+
2-
2-
Variable Valencies PLUMBITE PbO2
Cupric [Copper [II] ] Cu
2+
Argentic [Silver [II] ] Ag
2+
2+
Mercuric [Mercury [II] ] Hg
2+
Ferrous [Iron[II] ] Fe
2+
Plumbous [Lead [II] ] Pb
2+
Stannous [Tin [II] ] Sn
2+
Platinous [Platnium [II] ] Pt
2+
Manganous [Manganese [II] ] Mn
VALENCY 3-Trivalent elements VALENCY 3-Trivalent elements
3+
ALUMINIUM Al PHOSPHATE
3+
CHROMIUM Cr PO4
3-
3-
Variable Valencies NITRIDE N
Ferric [Iron [III] ] PHOSPHITE
3+
Fe PO3
3-
Auric [Gold [III] ]
Au
3+
VALENCY 4-Tetravalent elements VALENCY 4-Tetravalent elements
Variable Valencies CARBIDE
4-
Plumbic [Lead [IV] ] C
4+
Pb
Stannic [Tin [IV] ]
4+
Sn
Platinic [Platinum [IV] ] Pt
4+
4+
Manganic [Manganese [IV] ] Mn
Calculation of valency of ions: The valency of an ion is equal to the charge they
carry without the positive or negative sign
2+
Example: Calcium ion= Ca Valency = 2
+
Ammonium ion=NH4 Valency= 1
2