Page 2 - 3. LESSON NOTES-((3.1 MIND MAP & INTRODUCTION, LAWS OF CHEMICAL COMBINATIONS)L COMBINATIONS).docx
P. 2
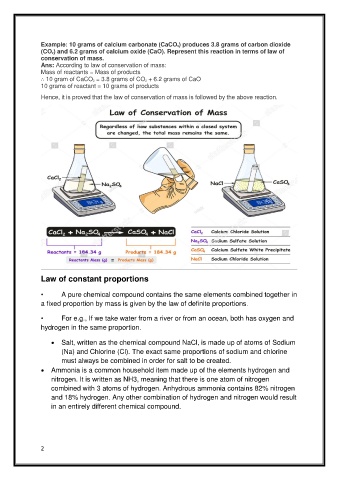
Example: 10 grams of calcium carbonate (CaCO 3) produces 3.8 grams of carbon dioxide
(CO 2) and 6.2 grams of calcium oxide (CaO). Represent this reaction in terms of law of
conservation of mass.
Ans: According to law of conservation of mass:
Mass of reactants = Mass of products
∴ 10 gram of CaCO 3 = 3.8 grams of CO 2 + 6.2 grams of CaO
10 grams of reactant = 10 grams of products
Hence, it is proved that the law of conservation of mass is followed by the above reaction.
Law of constant proportions
• A pure chemical compound contains the same elements combined together in
a fixed proportion by mass is given by the law of definite proportions.
• For e.g., If we take water from a river or from an ocean, both has oxygen and
hydrogen in the same proportion.
Salt, written as the chemical compound NaCl, is made up of atoms of Sodium
(Na) and Chlorine (Cl). The exact same proportions of sodium and chlorine
must always be combined in order for salt to be created.
Ammonia is a common household item made up of the elements hydrogen and
nitrogen. It is written as NH3, meaning that there is one atom of nitrogen
combined with 3 atoms of hydrogen. Anhydrous ammonia contains 82% nitrogen
and 18% hydrogen. Any other combination of hydrogen and nitrogen would result
in an entirely different chemical compound.
2