Page 1 - 3. Lesson Note-Difference between Elements and Compounds
P. 1
SAI International School
Subject-Chemistry
Ch-Is Matter around us pure?
Topic- Difference between elements compounds and mixtures
LESSON NOTE
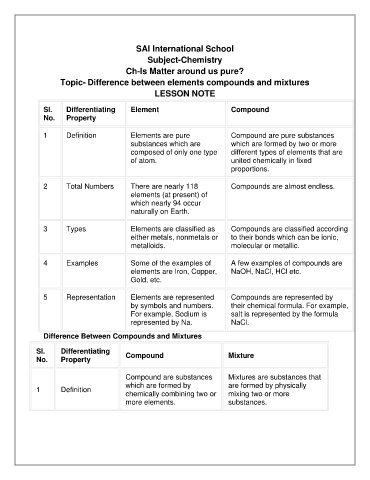
Sl. Differentiating Element Compound
No. Property
1 Definition Elements are pure Compound are pure substances
substances which are which are formed by two or more
composed of only one type different types of elements that are
of atom. united chemically in fixed
proportions.
2 Total Numbers There are nearly 118 Compounds are almost endless.
elements (at present) of
which nearly 94 occur
naturally on Earth.
3 Types Elements are classified as Compounds are classified according
either metals, nonmetals or to their bonds which can be ionic,
metalloids. molecular or metallic.
4 Examples Some of the examples of A few examples of compounds are
elements are Iron, Copper, NaOH, NaCl, HCl etc.
Gold, etc.
5 Representation Elements are represented Compounds are represented by
by symbols and numbers. their chemical formula. For example,
For example, Sodium is salt is represented by the formula
represented by Na. NaCl.
Difference Between Compounds and Mixtures
Sl. Differentiating Compound Mixture
No. Property
Compound are substances Mixtures are substances that
which are formed by are formed by physically
1 Definition
chemically combining two or mixing two or more
more elements. substances.