Page 2 - 3. LESSON NOTES-EFFECT OF PRESSURE ON STATES OF MATTER
P. 2
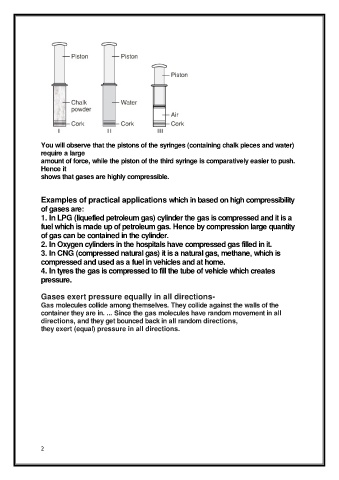
You will observe that the pistons of the syringes (containing chalk pieces and water)
require a large
amount of force, while the piston of the third syringe is comparatively easier to push.
Hence it
shows that gases are highly compressible.
Examples of practical applications which in based on high compressibility
of gases are:
1. In LPG (liquefied petroleum gas) cylinder the gas is compressed and it is a
fuel which is made up of petroleum gas. Hence by compression large quantity
of gas can be contained in the cylinder.
2. In Oxygen cylinders in the hospitals have compressed gas filled in it.
3. In CNG (compressed natural gas) it is a natural gas, methane, which is
compressed and used as a fuel in vehicles and at home.
4. In tyres the gas is compressed to fill the tube of vehicle which creates
pressure.
Gases exert pressure equally in all directions-
Gas molecules collide among themselves. They collide against the walls of the
container they are in. ... Since the gas molecules have random movement in all
directions, and they get bounced back in all random directions,
they exert (equal) pressure in all directions.
2