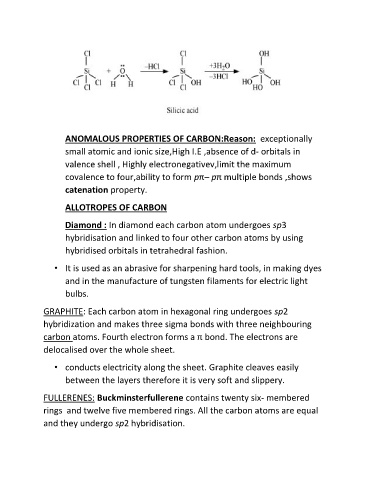
Page 2 - Microsoft Word - P-BLOCK ELEMENTS LN-4
P. 2
ANOMALOUS PROPERTIES OF CARBON:Reason: exceptionally
small atomic and ionic size,High I.E ,absence of d- orbitals in
valence shell , Highly electronegativev,limit the maximum
covalence to four,ability to form pπ– pπ multiple bonds ,shows
catenation property.
ALLOTROPES OF CARBON
Diamond : In diamond each carbon atom undergoes sp3
hybridisation and linked to four other carbon atoms by using
hybridised orbitals in tetrahedral fashion.
• It is used as an abrasive for sharpening hard tools, in making dyes
and in the manufacture of tungsten filaments for electric light
bulbs.
GRAPHITE: Each carbon atom in hexagonal ring undergoes sp2
hybridization and makes three sigma bonds with three neighbouring
carbon atoms. Fourth electron forms a π bond. The electrons are
delocalised over the whole sheet.
• conducts electricity along the sheet. Graphite cleaves easily
between the layers therefore it is very soft and slippery.
FULLERENES: Buckminsterfullerene contains twenty six- membered
rings and twelve five membered rings. All the carbon atoms are equal
and they undergo sp2 hybridisation.