Page 4 - 3. Lesson Notes Ch-5 MENDELEEVES CLASSIFICATION
P. 4
So, he could predict some of the elements to be discovered in future:
Mendeleev predicted the discovery of some elements and named them as-
a. eka-boron [Scandium,],
b. eka-aluminium [Gallium]
c. eka-silicon [Germanium].
He gave the name of these elements prefixing the word ‘eka’ to the name of the
preceding element.
2. Position of Noble gases which were discovered later:
Noble gases were discovered much later after Mendeleev formulated his periodic
table.
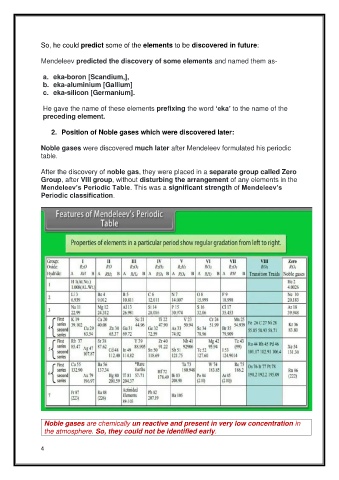
After the discovery of noble gas, they were placed in a separate group called Zero
Group, after VIII group, without disturbing the arrangement of any elements in the
Mendeleev’s Periodic Table. This was a significant strength of Mendeleev’s
Periodic classification.
Noble gases are chemically un reactive and present in very low concentration in
the atmosphere. So, they could not be identified early.
4