Page 2 - 2. HA- Obj & Sub Ch-5 CLASSIFICATION BY DOB.& NEWL.
P. 2
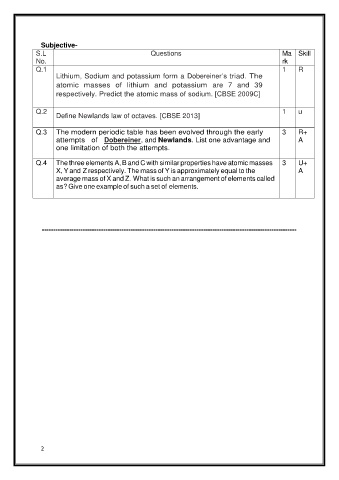
Subjective-
S.L Questions Ma Skill
No. rk
Q.1 1 R
Lithium, Sodium and potassium form a Dobereiner’s triad. The
atomic masses of lithium and potassium are 7 and 39
respectively. Predict the atomic mass of sodium. [CBSE 2009C]
Q.2 1 u
Define Newlands law of octaves. [CBSE 2013]
Q.3 The modern periodic table has been evolved through the early 3 R+
attempts of Dobereiner, and Newlands. List one advantage and A
one limitation of both the attempts.
Q.4 The three elements A, B and C with similar properties have atomic masses 3 U+
X, Y and Z respectively. The mass of Y is approximately equal to the A
average mass of X and Z. What is such an arrangement of elements called
as? Give one example of such a set of elements.
----------------------------------------------------------------------------------------------------------------
2