Page 7 - 3.LESSON NOTES-(CH-2 WASHING SODA& PLASTER OF PARIS)
P. 7
Water of Crystallization –
• Water of crystallisation is a fixed number of water molecules present in
one formula unit of a salt. Such salts are denoted as hydrated
compounds.
Example - One formula unit of copper sulphate contains five water molecules
(5H20). ...
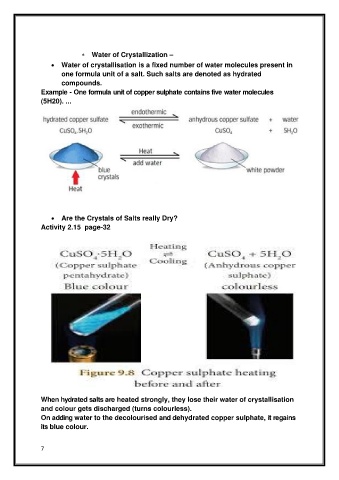
• Are the Crystals of Salts really Dry?
Activity 2.15 page-32
When hydrated salts are heated strongly, they lose their water of crystallisation
and colour gets discharged (turns colourless).
On adding water to the decolourised and dehydrated copper sulphate, it regains
its blue colour.
7