Page 1 - HA
P. 1
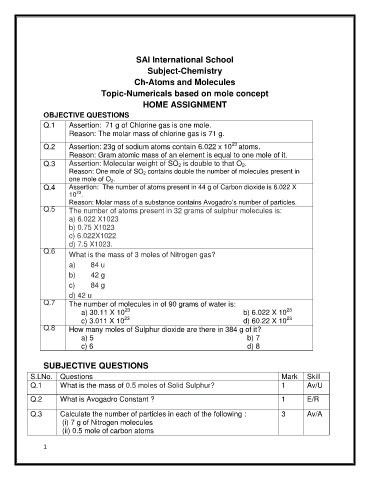
SAI International School
Subject-Chemistry
Ch-Atoms and Molecules
Topic-Numericals based on mole concept
HOME ASSIGNMENT
OBJECTIVE QUESTIONS
Q.1 Assertion: 71 g of Chlorine gas is one mole.
Reason: The molar mass of chlorine gas is 71 g.
23
Q.2 Assertion: 23g of sodium atoms contain 6.022 x 10 atoms.
Reason: Gram atomic mass of an element is equal to one mole of it.
Q.3 Assertion: Molecular weight of SO 2 is double to that O 2.
Reason: One mole of SO 2 contains double the number of molecules present in
one mole of O 2.
Q.4 Assertion: The number of atoms present in 44 g of Carbon dioxide is 6.022 X
23
10 .
Reason: Molar mass of a substance contains Avogadro’s number of particles.
Q.5 The number of atoms present in 32 grams of sulphur molecules is:
a) 6.022 X1023
b) 0.75 X1023
c) 6.022X1022
d) 7.5 X1023.
Q.6
What is the mass of 3 moles of Nitrogen gas?
a) 84 u
b) 42 g
c) 84 g
d) 42 u
Q.7 The number of molecules in of 90 grams of water is:
23
23
a) 30.11 X 10 b) 6.022 X 10
23
23
c) 3.011 X 10 d) 60.22 X 10
Q.8 How many moles of Sulphur dioxide are there in 384 g of it?
a) 5 b) 7
c) 6 d) 8
SUBJECTIVE QUESTIONS
S.LNo. Questions Mark Skill
Q.1 What is the mass of 0.5 moles of Solid Sulphur? 1 Av/U
Q.2 What is Avogadro Constant ? 1 E/R
Q.3 Calculate the number of particles in each of the following : 3 Av/A
(i) 7 g of Nitrogen molecules
(ii) 0.5 mole of carbon atoms
1