Page 2 - LN 4
P. 2
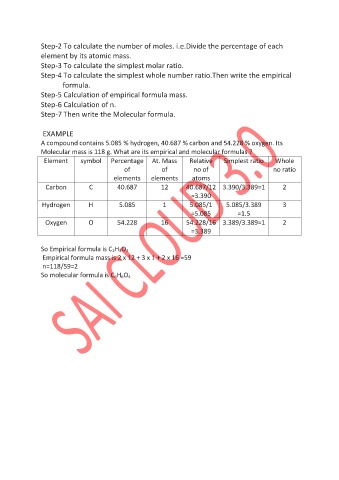
Step-2 To calculate the number of moles. i.e.Divide the percentage of each
element by its atomic mass.
Step-3 To calculate the simplest molar ratio.
Step-4 To calculate the simplest whole number ratio.Then write the empirical
formula.
Step-5 Calculation of empirical formula mass.
Step-6 Calculation of n.
Step-7 Then write the Molecular formula.
EXAMPLE
A compound contains 5.085 % hydrogen, 40.687 % carbon and 54.228 % oxygen. Its
Molecular mass is 118 g. What are its empirical and molecular formulas ?
Element symbol Percentage At. Mass Relative Simplest ratio Whole
of of no of no ratio
elements elements atoms
Carbon C 40.687 12 40.687/12 3.390/3.389=1 2
=3.390
Hydrogen H 5.085 1 5.085/1 5.085/3.389 3
=5.085 =1.5
Oxygen O 54.228 16 54.228/16 3.389/3.389=1 2
=3.389
So Empirical formula is C 2H 3O 2
Empirical formula mass is 2 x 12 + 3 x 1 + 2 x 16 =59
n=118/59=2
So molecular formula is C 4H 6O 4