Page 1 - 2.LESSON NOTES-CONCEPT MAP
P. 1
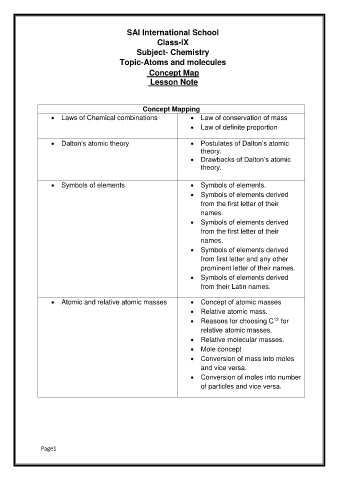
SAI International School
Class-IX
Subject- Chemistry
Topic-Atoms and molecules
Concept Map
Lesson Note
Concept Mapping
Laws of Chemical combinations Law of conservation of mass
Law of definite proportion
Dalton’s atomic theory Postulates of Dalton’s atomic
theory.
Drawbacks of Dalton’s atomic
theory.
Symbols of elements Symbols of elements.
Symbols of elements derived
from the first letter of their
names.
Symbols of elements derived
from the first letter of their
names.
Symbols of elements derived
from first letter and any other
prominent letter of their names.
Symbols of elements derived
from their Latin names.
Atomic and relative atomic masses Concept of atomic masses
Relative atomic mass.
12
Reasons for choosing C for
relative atomic masses.
Relative molecular masses.
Mole concept
Conversion of mass into moles
and vice versa.
Conversion of moles into number
of particles and vice versa.
Page1