Page 1 - 3. LESSON NOTES-(NUMERICALS BASED ON MOLE CONCEPT)
P. 1
SAI International School
Class-IX
Subject- Chemistry
Topic-Atoms and Molecules
Topic-Numericals based onMole concept
Lesson Note
Recaptulation:
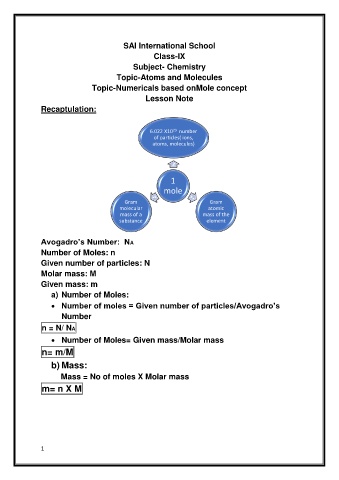
6.022 X10 23 number
of particles( ions,
atoms, molecules)
1
mole
Gram Gram
molecular atomic
mass of a mass of the
substance element
Avogadro’s Number: NA
Number of Moles: n
Given number of particles: N
Molar mass: M
Given mass: m
a) Number of Moles:
Number of moles = Given number of particles/Avogadro’s
Number
n = N/ NA
Number of Moles= Given mass/Molar mass
n= m/M
b) Mass:
Mass = No of moles X Molar mass
m= n X M
1