Page 1 - 4.HOME ASSIGNMENT-(MOLE CONCEPT)
P. 1
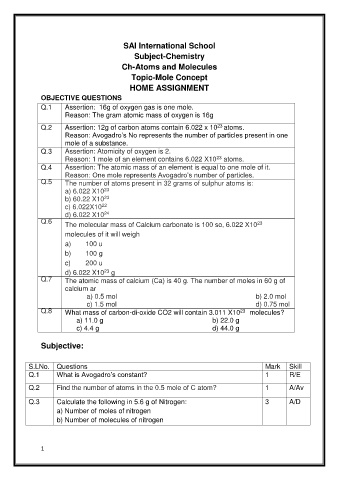
SAI International School
Subject-Chemistry
Ch-Atoms and Molecules
Topic-Mole Concept
HOME ASSIGNMENT
OBJECTIVE QUESTIONS
Q.1 Assertion: 16g of oxygen gas is one mole.
Reason: The gram atomic mass of oxygen is 16g
23
Q.2 Assertion: 12g of carbon atoms contain 6.022 x 10 atoms.
Reason: Avogadro’s No represents the number of particles present in one
mole of a substance.
Q.3 Assertion: Atomicity of oxygen is 2.
Reason: 1 mole of an element contains 6.022 X10 atoms.
23
Q.4 Assertion: The atomic mass of an element is equal to one mole of it.
Reason: One mole represents Avogadro’s number of particles.
Q.5 The number of atoms present in 32 grams of sulphur atoms is:
23
a) 6.022 X10
23
b) 60.22 X10
22
c) 6.022X10
24
d) 6.022 X10
Q.6
23
The molecular mass of Calcium carbonate is 100 so, 6.022 X10
molecules of it will weigh
a) 100 u
b) 100 g
c) 200 u
23
d) 6.022 X10 g
Q.7 The atomic mass of calcium (Ca) is 40 g. The number of moles in 60 g of
calcium ar
a) 0.5 mol b) 2.0 mol
c) 1.5 mol d) 0.75 mol
Q.8 What mass of carbon-di-oxide CO2 will contain 3.011 X10 molecules?
23
a) 11.0 g b) 22.0 g
c) 4.4 g d) 44.0 g
Subjective:
S.LNo. Questions Mark Skill
Q.1 What is Avogadro’s constant? 1 R/E
Q.2 Find the number of atoms in the 0.5 mole of C atom? 1 A/Av
Q.3 Calculate the following in 5.6 g of Nitrogen: 3 A/D
a) Number of moles of nitrogen
b) Number of molecules of nitrogen
1