Page 1 - 4.HOME ASSIGNMENT-(ATOMICITY& MOLECULAR MASS OF THE COMPOUNDS)
P. 1
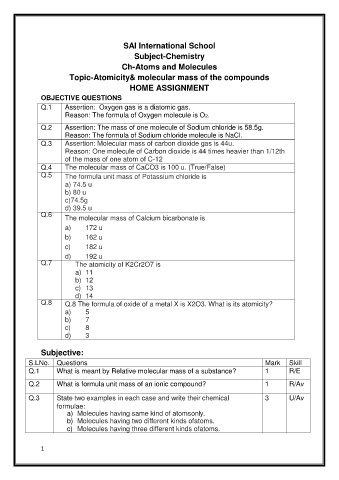
SAI International School
Subject-Chemistry
Ch-Atoms and Molecules
Topic-Atomicity& molecular mass of the compounds
HOME ASSIGNMENT
OBJECTIVE QUESTIONS
Q.1 Assertion: Oxygen gas is a diatomic gas.
Reason: The formula of Oxygen molecule is O2.
Q.2 Assertion: The mass of one molecule of Sodium chloride is 58.5g.
Reason: The formula of Sodium chloride molecule is NaCl.
Q.3 Assertion: Molecular mass of carbon dioxide gas is 44u.
Reason: One molecule of Carbon dioxide is 44 times heavier than 1/12th
of the mass of one atom of C-12
Q.4 The molecular mass of CaCO3 is 100 u. (True/False)
Q.5 The formula unit mass of Potassium chloride is
a) 74.5 u
b) 80 u
c)74.5g
d) 39.5 u
Q.6 The molecular mass of Calcium bicarbonate is
a) 172 u
b) 162 u
c) 182 u
d) 192 u
Q.7 The atomicity of K2Cr2O7 is
a) 11
b) 12
c) 13
d) 14
Q.8 Q.8 The formula of oxide of a metal X is X2O3. What is its atomicity?
a) 5
b) 7
c) 8
d) 3
Subjective:
S.LNo. Questions Mark Skill
Q.1 What is meant by Relative molecular mass of a substance? 1 R/E
Q.2 What is formula unit mass of an ionic compound? 1 R/Av
Q.3 State two examples in each case and write their chemical 3 U/Av
formulae:
a) Molecules having same kind of atomsonly.
b) Molecules having two different kinds ofatoms.
c) Molecules having three different kinds ofatoms.
1