Page 1 - 3. LESSON NOTES-(WRITING CHEMICAL FORMULAEOF THE COMPOUNDS)
P. 1
SAI International School
Class-IX
Subject- Chemistry
Topic-Atoms and Molecules
Topic-Writing chemical formulae of the compounds
LESSON NOTE
Recaptulation:
Ions
The charged particles (atoms) are called ions, they charge or negative charge on
it:
-
Negatively charged ion is called anion (Cl )
Positively charge ion is called cation (Na ).
+
Polyatomic ions: These are the ions which contain atoms of more than one element
which carry a single charge and behave as a single unit in chemical combinations.
2-
+
Example: NH4 ,SO4
• Valency
The combining capacity of an element is known as its valency:
Writing formula of compounds:
Compounds
• When two or more elements chemically combine in a fixed ratio by mass, the
obtained product is known as a compound.
• Compounds are substances consisting of two or more different types of
elements in a fixed ratio of its atoms.
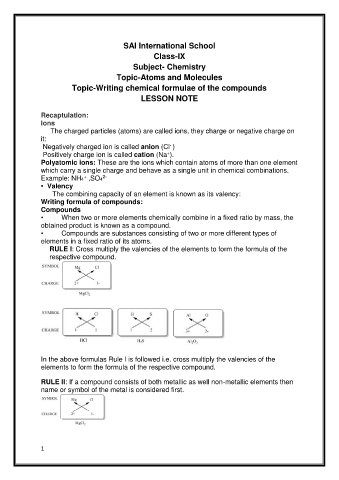
RULE I: Cross multiply the valencies of the elements to form the formula of the
respective compound.
In the above formulas Rule I is followed i.e. cross multiply the valencies of the
elements to form the formula of the respective compound.
RULE II: If a compound consists of both metallic as well non-metallic elements then
name or symbol of the metal is considered first.
1