Page 1 - 4. HOME ASSIGNMENT-(IONSPOSITIVE&NEGATIVE IONSCALCULATION OF VALENCY OF IONS)
P. 1
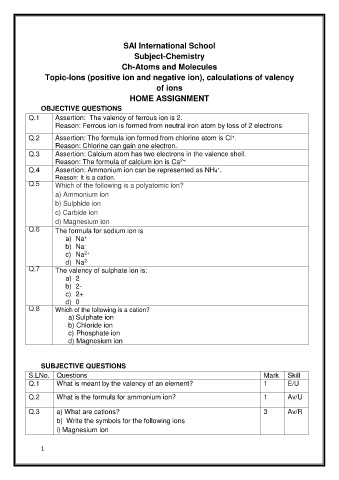
SAI International School
Subject-Chemistry
Ch-Atoms and Molecules
Topic-Ions (positive ion and negative ion), calculations of valency
of ions
HOME ASSIGNMENT
OBJECTIVE QUESTIONS
Q.1 Assertion: The valency of ferrous ion is 2.
Reason: Ferrous ion is formed from neutral iron atom by loss of 2 electrons
+
Q.2 Assertion: The formula ion formed from chlorine atom is Cl .
Reason: Chlorine can gain one electron.
Q.3 Assertion: Calcium atom has two electrons in the valence shell.
2+.
Reason: The formula of calcium ion is Ca
+
Q.4 Assertion: Ammonium ion can be represented as NH4 .
Reason: It is a cation.
Q.5 Which of the following is a polyatomic ion?
a) Ammonium ion
b) Sulphide ion
c) Carbide ion
d) Magnesium ion
Q.6 The formula for sodium ion is
+
a) Na
-
b) Na
c) Na
2+
d) Na
2-
Q.7 The valency of sulphate ion is:
a) 2
b) 2-
c) 2+
d) 0
Q.8 Which of the following is a cation?
a) Sulphate ion
b) Chloride ion
c) Phosphate ion
d) Magnesium ion
SUBJECTIVE QUESTIONS
S.LNo. Questions Mark Skill
Q.1 What is meant by the valency of an element? 1 E/U
Q.2 What is the formula for ammonium ion? 1 Av/U
Q.3 a) What are cations? 3 Av/R
b) Write the symbols for the following ions
i) Magnesium ion
1