Page 1 - 3. LESSON NOTES-(SYMBOLS OF ELEMENTS)
P. 1
SAI International School
Subject-Chemistry
Ch-Atoms and Molecules
Topic-Symbols of elements
LESSON NOTE
Atoms: These are the smallest particle of matter which may or may not have an
independent existence and take part in chemical reactions.
Molecules: These are the smallest particle of matter which can exist
independently.
Atoms:
The smallest tiny particles of matter which can't be divided further is called atom, i.e.,
an atom is the smallest building block of matter.
For example: Sodium (Na), Hydrogen (H), Oxygen (O), etc.
Names of Elements and their Symbols:
John Dalton in 1808 first used figurative symbols to represent different elements. As
you can see it is tedious and non-practical hence it was soon abandoned
IUPAC (International Union of Pure and Applied Chemistry) approves names of
elements.
The abbreviation used for lengthy names of elements are termed as their symbols.
The symbol of an element is formed by writing only the first letter or first letter
followed by the second or some other letter of English name or Latin name of the
element.
While writing a symbol, the first letter is always capital and the second is always
small.
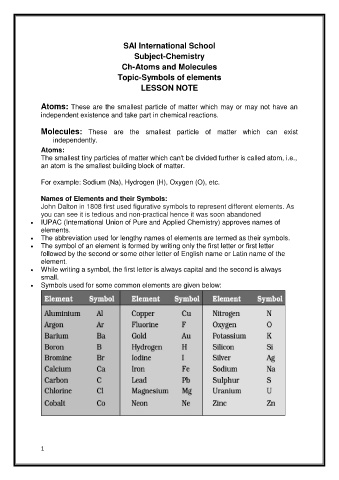
Symbols used for some common elements are given below:
1