Page 1 - 3. LESSON NOTES-DIFFERENT STATES OF MATTER
P. 1
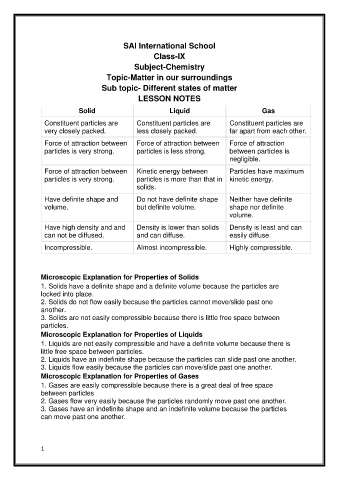
SAI International School
Class-IX
Subject-Chemistry
Topic-Matter in our surroundings
Sub topic- Different states of matter
LESSON NOTES
Solid Liquid Gas
Constituent particles are Constituent particles are Constituent particles are
very closely packed. less closely packed. far apart from each other.
Force of attraction between Force of attraction between Force of attraction
particles is very strong. particles is less strong. between particles is
negligible.
Force of attraction between Kinetic energy between Particles have maximum
particles is very strong. particles is more than that in kinetic energy.
solids.
Have definite shape and Do not have definite shape Neither have definite
volume. but definite volume. shape nor definite
volume.
Have high density and and Density is lower than solids Density is least and can
can not be diffused. and can diffuse. easily diffuse.
Incompressible. Almost incompressible. Highly compressible.
Microscopic Explanation for Properties of Solids
1. Solids have a definite shape and a definite volume because the particles are
locked into place.
2. Solids do not flow easily because the particles cannot move/slide past one
another.
3. Solids are not easily compressible because there is little free space between
particles.
Microscopic Explanation for Properties of Liquids
1. Liquids are not easily compressible and have a definite volume because there is
little free space between particles.
2. Liquids have an indefinite shape because the particles can slide past one another.
3. Liquids flow easily because the particles can move/slide past one another.
Microscopic Explanation for Properties of Gases
1. Gases are easily compressible because there is a great deal of free space
between particles
2. Gases flow very easily because the particles randomly move past one another.
3. Gases have an indefinite shape and an indefinite volume because the particles
can move past one another.
1