Page 1 - HA1
P. 1
SAI INTERNATIONAL SCHOOL
CLASS - VIII
Subject – CHEMISTRY
Chapter – CHEMICAL EFFECTS OF ELECTRIC CURRENT
TOPIC – Good and Bad Conductors, Do Liquids Conduct Electricity?
HOME ASSIGNMENT
Objective:
Q. No. Questions
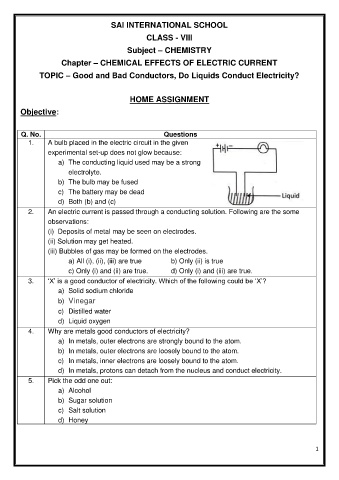
1. A bulb placed in the electric circuit in the given
experimental set-up does not glow because:
a) The conducting liquid used may be a strong
electrolyte.
b) The bulb may be fused
c) The battery may be dead
d) Both (b) and (c)
2. An electric current is passed through a conducting solution. Following are the some
observations:
(i) Deposits of metal may be seen on electrodes.
(ii) Solution may get heated.
(iii) Bubbles of gas may be formed on the electrodes.
a) All (i), (ii), (iii) are true b) Only (ii) is true
c) Only (i) and (ii) are true. d) Only (i) and (iii) are true.
3. ‘X’ is a good conductor of electricity. Which of the following could be ‘X’?
a) Solid sodium chloride
b) Vinegar
c) Distilled water
d) Liquid oxygen
4. Why are metals good conductors of electricity?
a) In metals, outer electrons are strongly bound to the atom.
b) In metals, outer electrons are loosely bound to the atom.
c) In metals, inner electrons are loosely bound to the atom.
d) In metals, protons can detach from the nucleus and conduct electricity.
5. Pick the odd one out:
a) Alcohol
b) Sugar solution
c) Salt solution
d) Honey
1