Page 1 - HA
P. 1
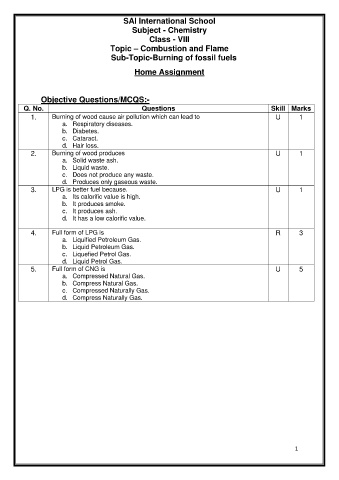
SAI International School
Subject - Chemistry
Class - VIII
Topic – Combustion and Flame
Sub-Topic-Burning of fossil fuels
Home Assignment
Objective Questions/MCQS:-
Q. No. Questions Skill Marks
1. Burning of wood cause air pollution which can lead to U 1
a. Respiratory diseases.
b. Diabetes.
c. Cataract.
d. Hair loss.
2. Burning of wood produces U 1
a. Solid waste ash.
b. Liquid waste.
c. Does not produce any waste.
d. Produces only gaseous waste.
3. LPG is better fuel because. U 1
a. Its calorific value is high.
b. It produces smoke.
c. It produces ash.
d. It has a low calorific value.
4. Full form of LPG is R 3
a. Liquified Petroleum Gas.
b. Liquid Petroleum Gas.
c. Liquefied Petrol Gas.
d. Liquid Petrol Gas.
5. Full form of CNG is U 5
a. Compressed Natural Gas.
b. Compress Natural Gas.
c. Compressed Naturally Gas.
d. Compress Naturally Gas.
1