Page 1 - KTG-MM-#141513130101
P. 1
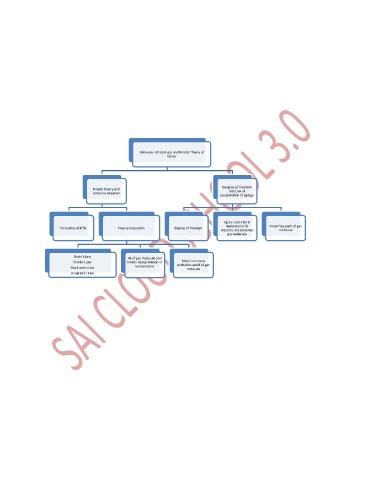
Behaviour of ideal gas and Kinetic Theory of
Gases
Deegree of freedom
Kinetic theory and
pressure equation and Law of
equipartition of egergy
Cp,Cv. and for i)
monoatomic ii) mean free path of gas
Postulates of KTG Pressure equation Degree of freedom
diatomic. iii) triatomic molecule
gas molecule
Boyle's Law
KE of gas molecule and
Mean.rms.most
Charle's Law kinetic interpretation of probable speed of gas
Gay Lusac's Law temperature
molecule
Avogrado's Law