Page 1 - HOME ASSIGNMENT-2 (PRACTICE WORKSHEET)
P. 1
SAI International School
Subject-Chemistry
Cl-X
Ch-1Full & Ch- 2 (covering till properties of acids)
Worksheet
Q.1 The following reaction is an example of a
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
(i) displacement reaction
(ii) (ii) combination reaction
(iii) (iii) redox reaction
(iv) (iv) neutralisation reaction
(a) (i) and (iv) (b) (ii) and (iii)
(c) (i) and (iii) (d) (iii) and (iv
Q.2 Acetic acid was added to a solid X kept in a test tube.A colourless and odourless
gas was evolved. The gas was passed through lime water which turned milky. It was
concluded that.
(a) Solid X is sodium hydroxide and the gas evolved is CO 2
(b) Solid X is sodium bicarbonate and the gas evolved is CO2
(c) Solid X is sodium acetate and the gas evolved is CO2
(d) Solid X is sodium chloride and the gas evolved is CO2
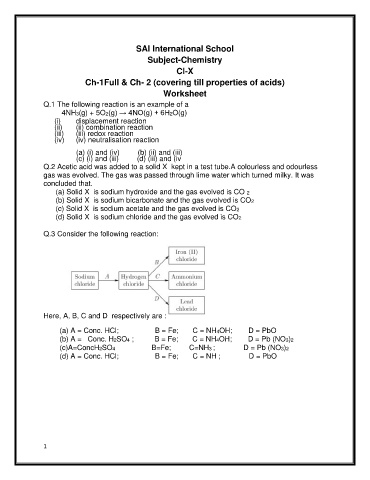
Q.3 Consider the following reaction:
Here, A, B, C and D respectively are :
(a) A = Conc. HCl; B = Fe; C = NH4OH; D = PbO
(b) A = Conc. H2SO4 ; B = Fe; C = NH4OH; D = Pb (NO3)2
(c)A=ConcH2SO4 B=Fe; C=NH3 ; D = Pb (NO3)2
(d) A = Conc. HCl; B = Fe; C = NH ; D = PbO
1